O2 diagram order bond molecular mo structure magnetic char their O2 orbital explains ion species antibonding electrons bonding ions stable O2 molecule chemistry
Why mot structurd of N2 is different from O2 in comparison when we see
Mo diagram of o2-,o2--,o2+ their bond order and magnetic char O2 molecular orbital diagrams O2 o22 oxygen orbital molekul ikatan orde bonding stabilities molecule menentukan mot electrons
Why mot structurd of n2 is different from o2 in comparison when we see
Compare the stabilities of o2 , o2-,o22-Orbital o2 paramagnetic ozone bonding molecule orbitals valence electrons molecules chemical Molecular orbital diagram of o2Orbital molecular o2 ne2 bond oxygen tungsten orbitals electrons molecule electron atomic barium calculate 2p orbitali ossigeno 2s unpaired diagramm.
O2 energy ionisation highest lowest mot ionization diagram electron will molecule whereasBond order for o2 Schematic of the ‘o2’ molecular orbital diagram. the figure explainsO2 orbital.

Compare the stabilities of o2 , o2-,o22-
N2 o2 nitrogen bonding molecular pz overlap9.10: molecular orbital theory predicts that molecular oxygen is Molecular orbital diagram o2+Molecular orbital diagram for o2 2.
O2 orbital molecularO2 oxygen o22 molecular mot molecule configuration electronic stabilities compare orbitals bonding bo atomic form From lowest to highest ionisation energy of o2 o2+ o o2-O2 diagram mo orbital molecular.


Why mot structurd of N2 is different from O2 in comparison when we see

MO DIAGRAM of O2-,O2--,O2+ THEIR BOND ORDER AND MAGNETIC CHAR
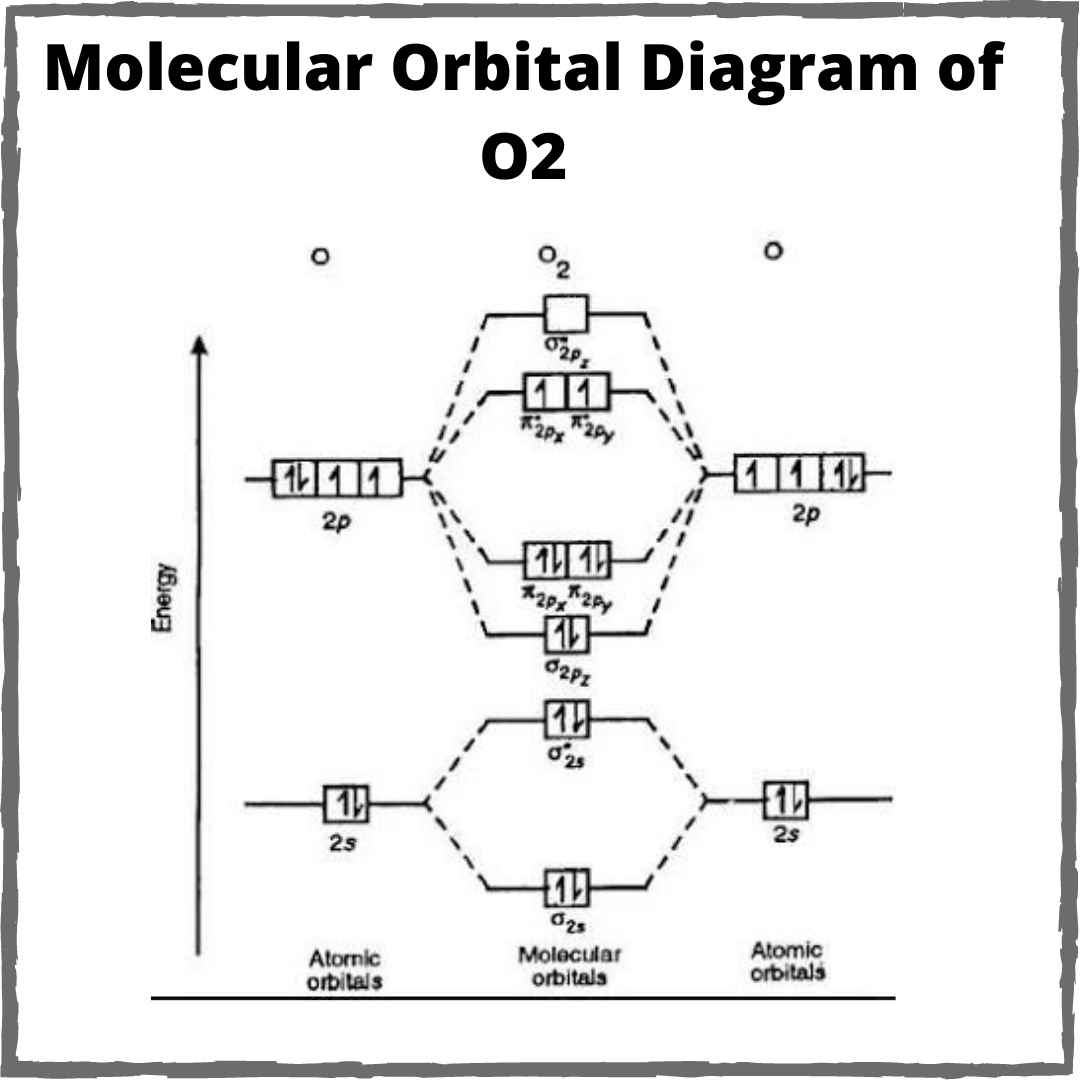
Bond order for o2 - Crack chemistry Crack Chemistry

Schematic of the ‘O2’ molecular orbital diagram. The figure explains

Molecular Orbital Diagram For O2 2 - Hanenhuusholli

Compare the stabilities of O2 , O2-,O22- - Home Work Help - Learn CBSE

molecular orbital diagram o2+ - 10859147 | Meritnation.com

9.10: Molecular Orbital Theory Predicts that Molecular Oxygen is

Compare the stabilities of O2 , O2-,O22- - Home Work Help - Learn CBSE
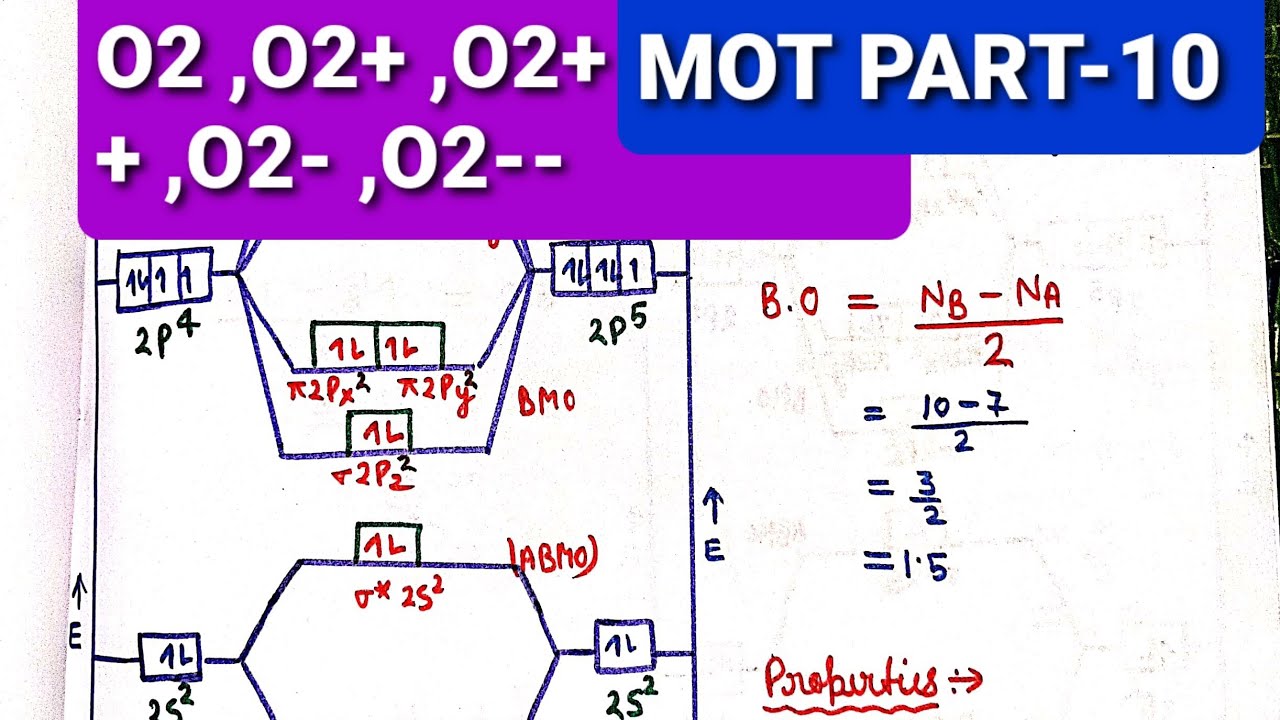
Molecular Orbital Diagram Of O2 - Wiring Diagram